Discover how Videojet Plays an Important Role in Marking and Coding Requirements for the Pharmaceutical and
Medical Industries!

Our contribution in the fight against COVID-19: Coding masks, diagnostics, vials and syringes
Videojet responds to the increasing demand for personal protective equipment (PPE), diagnostics, vials, and syringes by shifting its technologies and resources to meet the needs of essential manufacturers during these challenging times. Watch the video and learn how Videojet can help in the fight against COVID-19.

Code2Carton: Tested marking quality for cartons
When it comes to traceability, it is crucial that the codes on pharmaceutical products remain readable over the long term. Yet influences along the distribution chain such as condensation or UV radiation may blur or fade the code.
In order to help ensure optimum marking quality on carton packaging, Videojet has worked together with the Paper Technology Foundation (PTS) to offer a test for Videojet codes on folding cartons.
Learn more about Videojet Code2Carton here.

Videojet Pharma Line: Serialization and track & trace compliance
To stop counterfeits, new regulations are being introduced around the world to implement serialization and track & trace programs. Find out more about how Videojet can help!
Learn more about global coding requirements related to serialization regulations here.
Learn more about coding regulations on medical devices here.
Videojet Offers Solutions for the Changing and Challenging Marking and Coding Needs
for the Pharmaceutical and Medical Industries.

Medical masks have become an essential item in our daily lives in the fight against COVID-19. Medical masks are considered as personal protective equipment (PPE), and are therefore classified as medical devices. They are subject to local regulations such as the US UDI system, or the European Medical Device Regulation (MDR), and must be labeled accordingly. Videojet can help medical mask manufacturers comply with these legal requirements by offering innovative coding solutions for masks, packaging and shipping boxes.
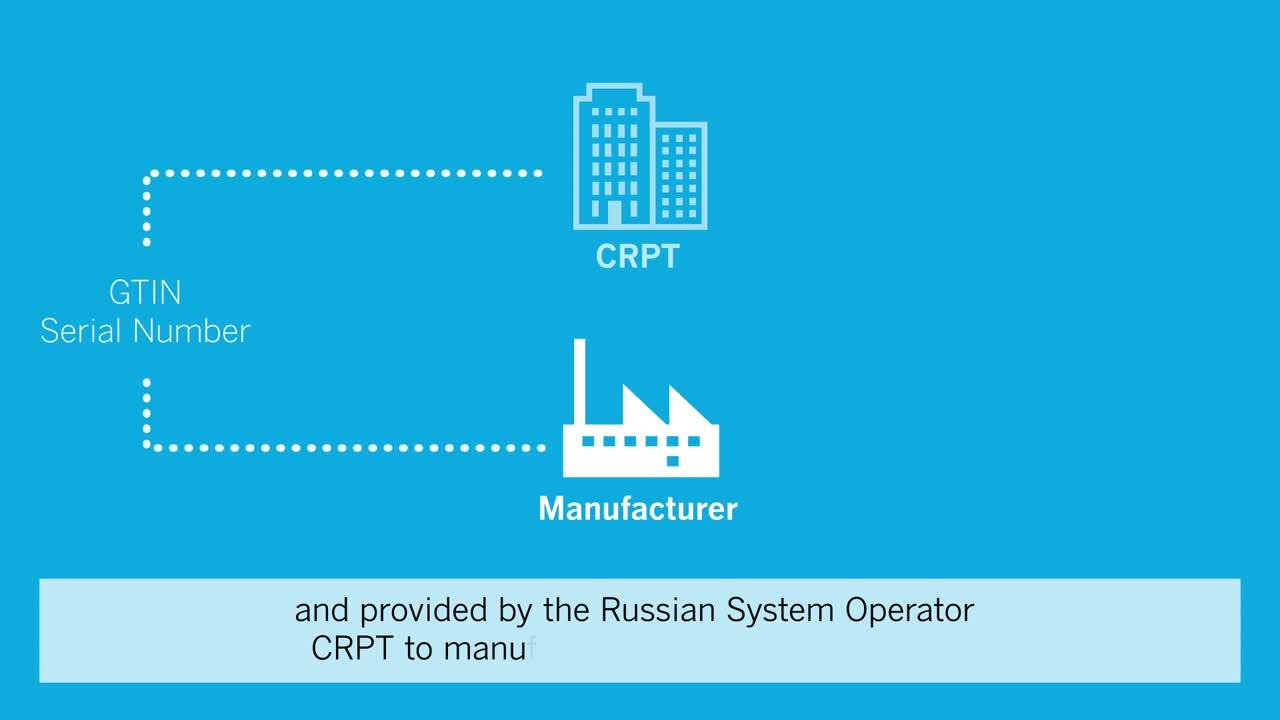
Effective as of January 1, 2019, the Russian Federal Act No. 488-FZ mandates that 12 different product groups marketed in Russia, including pharmaceuticals, require traceability serialization using a Crypto-Code no later than July 1, 2020.
Videojet released a software update for the Wolke m610/m600 series to optimize Crypto-Code printing.

Perhaps to a greater degree than other industries, pharmaceutical and medical device packaging demands the highest quality variable coding.
Therefore, Videojet can provide IQ/OQ validation packages for coding solutions to help meet your documentation needs.
Learn More About Unique Solutions Offered by Videojet for Pharmaceutical and Medical Needs From our Expert!

Coding pharmaceutical cartons with Wolke thermal inkjet or Videojet 3000 series CO2 laser marking systems.
Videojet Pharma Line offers end-to-end solutions for pharmaceutical marking and coding. The video demonstrates two options on how to meet the regulations for applying 2D serialized codes on cartons using Wolke thermal inkjet or Videojet 3000 Series CO2 laser marking systems.
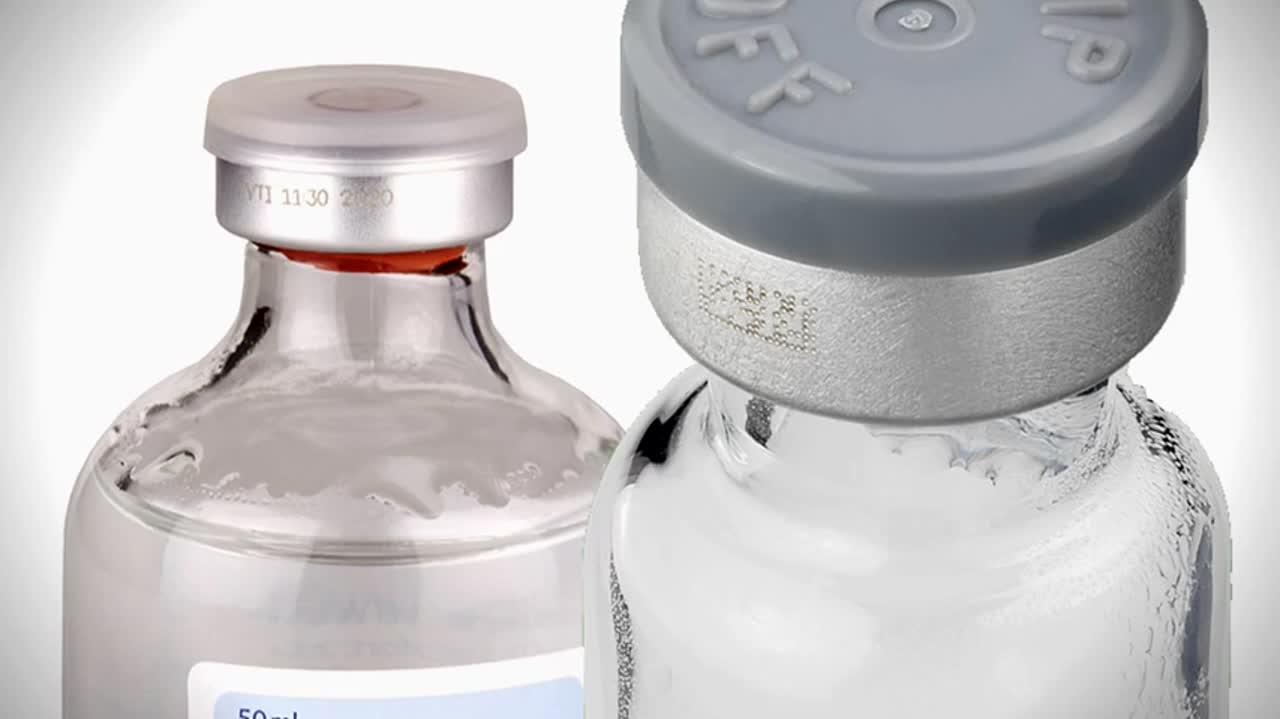
Coding pharmaceutical vials with Videojet 7510 fiber laser or Videojet continuous inkjet (CIJ) printers.
Videojet Pharma Line offers end-to-end solutions for pharmaceutical marking and coding. The video demonstrates two options on how to mark 2D bar codes on vial caps in an FP Development Starwheel Vial Coding and Verifying Machine – either using a Videojet 7510 laser or Videojet continuous inkjet (CIJ) printer.
To know more or get expert advice for marking and coding requirements on pharmaceuticals or medical products, contact us.

Applying coded labels with the Videojet 9550 Label Printer Applicator (LPA).
Videojet Pharma Line offers end-to-end solutions for pharmaceutical marking and coding. The video demonstrates on how to apply serialized codes on cartons with the Videojet 9550 Label Printer Applicator (LPA) 9550.
Learn More About Videojet Solutions for Pharmaceutical and Medical Device Requirements Below.

“Increasing code quality and efficiency in serialization” – Presentation at Pharmapack 2018, Paris
This 25-minute presentation, delivered by Heidi Vanheerswynghels, outlines the key considerations manufacturers should acknowledge in order to achieve high-quality bar codes that meet the requirements of the European Falsified Medicine Directive.
At the end of this video you’ll understand:
- Global serialization legislations
- Key influences on bar code quality
- ISO 15415 – Parameters measured, causes and optimizations
- Testing quality – How do you ensure and test the quality of codes?
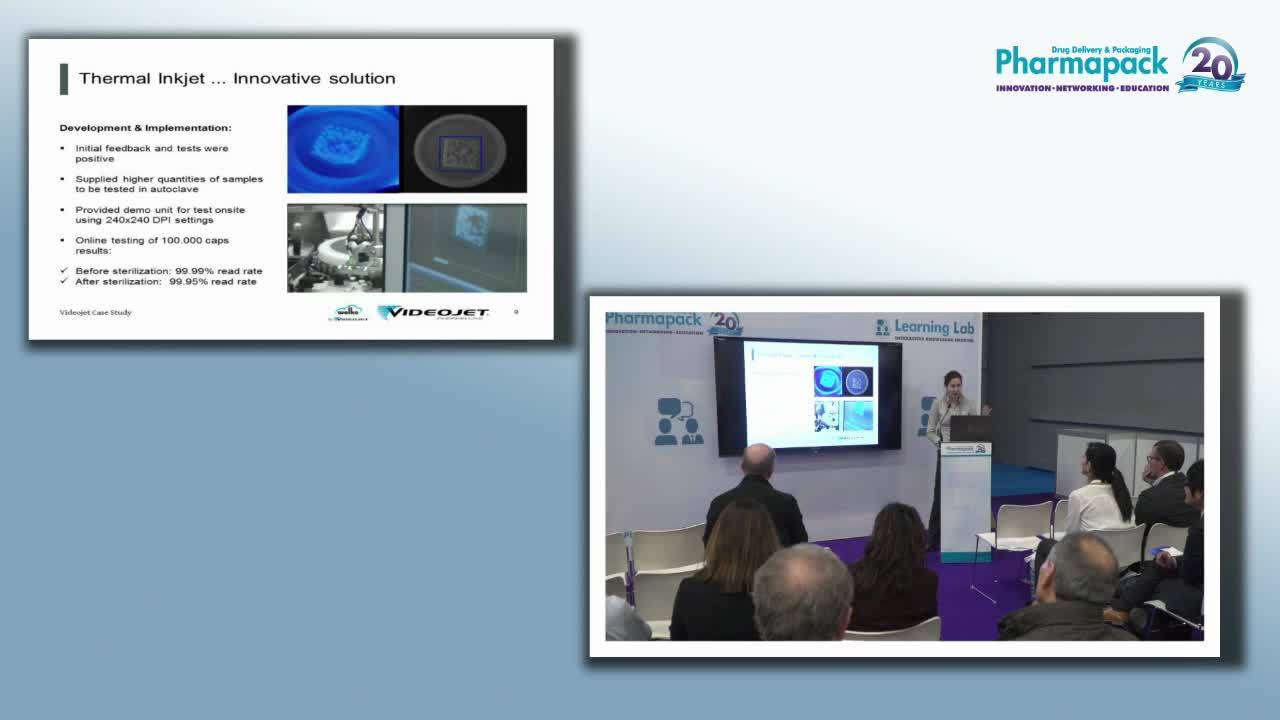
“Invisible vial coding with Wolke thermal inkjet” – A case study presented at Pharmapack 2017, Paris
This 16-minute presentation, delivered by Heidi Vanheerswynghels at Pharmapack 2017, shows how vial caps are coded with a UV thermal inkjet ink to help ensure internal traceability.
Fill in your details below to receive latest updates from Videojet
Know more about Industry Case studies about Videojet!

Case Study: Bosch Packaging Technology
Find out how Bosch utilizes the Wolke m610 OEM for a powerful track and trace solution.
Click below to View more!